[FELIX-1 White Paper] – Computational Fluid Dynamic Modelling of Particle Capture: Possible Use of a Modified Face-Tent as a Facial Scavenger
Scott Leonard, BSME1; George C. Dungan II, MPhil(Med)1,2,3
1Vapotherm, Inc. Exeter, NH, USA; 2NHMRC Centre for Integrated Research and Understanding of Sleep-CIRUS, Australia; 3Education and Human Services, Canisius College, Buffalo, NY USA.
ABSTRACT
Aerosol generating procedures (AGPs) present a substantial clinical challenge during any clinical interaction, much less during a global viral pandemic. Control of AGPs during respiratory therapy procedures is important. Various methods of mitigation have been proposed for non-invasive ventilatory support. This study evaluated the use of an area of relative negative pressure at the face of the patient, achieved by applying suction flow to a modified face-tent mask, using computational fluid dynamic modeling.
AGPs associated with High Flow therapy continues to be a concern. An in silico modeling study of High Velocity Nasal Insufflation (HVNI) at 40 L∙min-1 with a simulated surgical mask suggests the capture of exhaled particulate mass in the mask of 88.8%. A computational fluid dynamic (CFD) model depicting a system including a face-tent modified to create an area of relative negative pressure (FELIX-1) was tested. The CFD model included anatomically correct rendering of a human head and airway, as well as a simulated FELIX-1 system depicted correctly positioned on the head model, along with a simulated HVNI cannula. The model included dynamics corresponding to patient breathing whilst receiving 40L∙min-1 of HVNI. The simulation showed up to 97% of the particle mass leaving the patient whilst being captured into the mask or removed by the suction system. The model suggests that the amount of suction-generated airflow plays a role in the efficacy of potential particle capture. The simulation also showed the potential for substantial reduction in temperature immediately proximate to the face of the model with the FELIX-1 during HVNI therapy, as compared to that achieved with the use of a simple surgical facemask similarly modeled. This simulation suggests the FELIX-1, when properly fitted and under adequate suction-generated airflow, may play a role in helping to reduce the burden of particle mass dispersal during High Flow therapy. This model does not suggest that the use of a configuration such as FELIX-1 would obviate the need for all standard precautions during patient management, including use of personal protective equipment (PPE) and the use of negative pressure rooms when available. Future in vitro or in vivo simulations may be appropriate.
INTRODUCTION
Infectious disease transmission by airborne particles presents a serious challenge in management of respiratory viral disease. COVID-19, the clinical disease related to infection with the SARS-CoV-2 coronavirus (COVID-19) is associated with varying degrees of respiratory distress, hypoxemia, and respiratory failure. The challenges of management of these patients’ oxygen
has been addressed in recent guidelines from numerous agencies and organizations. [1] [2] [3] [4] These guidance documents all include the use of High Flow Oxygen (HFO) therapy in the list of possible early interventions, while emphasizing caution regarding the potential aerosol generation from any non-invasive respiratory therapy. Although true aerosol airborne particles (i.e., <5μm) represent a possible vector for transmission of the virus, the World Health Organization (WHO) has stated and reconfirmed that the primary mode of transmission is via respiratory droplets (those ≥5μm). [5] [6] All guidelines clearly state and reinforce the requirement for strict adherence to PPE guidelines and use of environmental controls, including negative pressure rooms, when available.
Aerosol generating procedures (AGPs) produce potentially infectious particles which can enter the environment around an infected patient. AGPs include most forms of respiratory care intervention, and serve as a potential source of nosocomial spread of viral disease, as AGPs create the potential for airborne transmission of infections that may otherwise only be transmissible by the droplet route. [7]
Known AGPs include intubation, extubation and related procedures such as manual ventilation and open suctioning, bronchoscopy, Non-Invasive Ventilation (NIV), High-Frequency Oscillating Ventilation (HFOV), High Flow Oxygen (including High Flow Nasal Cannula), induction of sputum, and any procedure that induces coughing. [8] [9] Particle sizes of aerosols or droplets can directly affect transmission distance, along with room geometry and air-movement, entry and activity in the room, and purposeful negative pressures. [10-14]
High Flow Nasal Cannula (HFNC) and High Velocity Nasal Insufflation (HVNI) therapy share a common characteristic, namely the administration of oxygen-rich gas to a patient using an open interface. HVNI is a form of NIV which delivers High Flow Oxygen (similar to HFNC) with the addition of imparting high velocity to the heated/humidified gas mixture as it leaves a simple cannula interface. This advantage is particularly important when considering the ability to flush the accessible extrathoracic deadspace and provide not only oxygenation but ventilatory support to spontaneously breathing patients.
While there have been questions about High Flow therapies presenting an infection risk, existing data suggest that HFNC is a relatively low-ranking source of infectious particle dispersal as long as good affixation and placement practices are followed. [15] [8] [16] [17] Other non-invasive forms of oxygen and ventilatory support present additional potential challenges, as in the case of placing an Non-Invasive Positive Pressure Ventilation (NiPPV) mask-to-skin interface. [16] [17]
Work to evaluate the mitigation of even residual risk of particle dispersal has led to recent suggestions for adjunctive approaches concomitantly applied during HFNC therapy, including the addition of a simple surgical mask over a High Flow therapy interface as a mitigation to particle dispersal.3 Such a configuration was evaluated using a computational fluid dynamic modeling approach, and suggested, in that model, that a substantial proportion of the particulate mass could be effectively captured. [18]
Such an approach has the advantage of simple implementation but is not without possible limitations to effectual clinical deployment (including mask saturation and uncomfortable warmth under the mask). An alternative approach was evaluated after suggestion from Felix Khusid RRT-ACCS,NPS, RPFT, FAARC, FCCM, FCCP, ATSF, Administrative Director of Respiratory Therapy and Pulmonary Physiology in New York; the use of a modified face-tent mask, connected to a hospital vacuum line, for the purpose of providing a region of relative negative pressure at the face of a patient with the goal of ‘scavenging’ particulate mass from the region of the face. Such an embodiment has several potential advantages: open comfortable architecture, reduced temperature at the face of the patient compared to a surgical mask, and a durable device for long-term use by the patient. This study will evaluate the particle capture potential of the FELIX-1 embodiment using computational fluid dynamic (CFD) methodologies.
METHODS
Description of the Modeled Embodiment
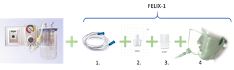
In order to establish a meaningful simulation, a physical embodiment of a modified face-tent mask (FELIX-1, Vapotherm, Inc, Exeter, NH, USA) was assembled and tested mechanically as a plausible and manufacturable design. This model included the following components (Figure 1):
- 6mm Suction tubing with length of 2m or less
- 8mm to 22mm adapter (part number 52208, Qosina, Ronkonkoma, NY, USA)
- 22mm female to female coupling (part number 51487, Qosina, Ronkonkoma, NY, USA)
- Face-tent mask with 22mm male fitting
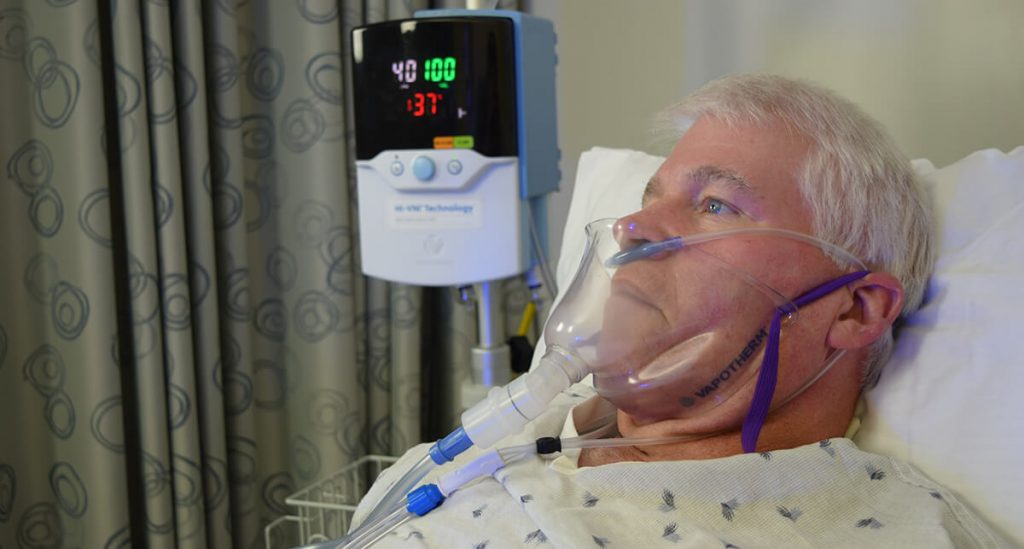
When placed on the patient, the FELIX-1 will rest snugly but comfortably under the chin of the patient (Figure 2).
Computational Fluid Dynamic Model
Model Geometry, Assumptions, and Conditions

A CAD model of a face-tent mask is placed on a Computed Tomography (CT) derived 3D model of a human head with a Vapotherm Adult Sm. / Pediatric Cannula. (Figure 3)
Face-tent model: An accurately rendered standard face-tent was modeled using measurements and reference images taken from a commercially-available face-tent (part number OM-81312, Besmed, New Taipei City, Taiwan). The model was adapted to achieve a tight fit to the head model contours. The modeling is similar to that previously described. [18]
Cannula Model: An accurate depiction of the geometry and dimensions of the Vapotherm Adult Small / Pediatric cannula nose piece is included in the model, positioned with its non-occlusive prongs in the patient’s nares. Flow of therapeutic gas is defined to be emitted from the prongs of the cannula.
Particle Model: The distribution of particle sizes emitted by a patient breathing, talking, coughing, and sneezing has been investigated in several studies. While the specific distributions vary, the range of particle sizes that are meaningful to study is generally from 0.1 to 100µm. Particles larger than 100µm are highly unlikely to penetrate a mask or travel far without meaningful velocity. Particles smaller than 0.1µm account for a very small fraction of the total particles, are likely to escape regardless of a mask.
Such particles have not been demonstrated to be associated with transmission.6
The particle distribution for the simulation is taken from exhaled droplets due to talking and coughing. [13] A Rosin-Rammler Diameter distribution method is used in the simulation to generate particles that approximate the particle distribution. Particles smaller than 0.1µm and larger than 100µm were not included. The Rosin-Rammler parameters, d ̅=70 and n = 0.990 are used for the simulation (Table 1).
Flow Conditions
In all of the cases, the patient is modeled to be breathing at 32 breaths per minute with a simulated tidal volume of 500mL. The breath curve is sinusoidal with a 1:1 inspiratory to expiratory ratio and no pause between the inspiratory and expiratory phases. The cannula flow rate for HVNI therapy is 40 L∙min-1. Suction flow rates greater than 20 L∙min-1 are expected to be achieved with standard hospital suction systems. For the simulation, additional suction flow rates covering a range from 1 to 80 L∙min-1 were modeled to allow understanding of the sensitivity of efficiency to flow rate.
Simulation
The simulations are transient, accounting for variation of the flow with time caused by cyclic breathing. Using a time step of 0.001s, the simulations run for several breath cycles until quasi-steady-state flow is achieved. Particles are emitted into the flow at a time step corresponding to peak expiratory flow rate. The particles are tracked through their trajectories and their final position is reported. The particles are grouped into one of the following categories:
| Used in Simulation | Used in Curve Fit (not simulated) | ||
| Particle Size (µm) | Mass Fraction (%) | Particle Size (µm) | Mass Fraction (%) |
| 0 – 5 | 2.7 | 100 – 150 | 11.3 |
| 5 – 10 | 12.2 | 150 – 200 | 3.1 |
| 10 – 15 | 8.5 | 200 – 250 | 2.4 |
| 15 – 20 | 4.5 | 250 – 300 | 0.7 |
| 20 – 25 | 3.9 | 300 – 350 | 2 |
| 25 – 30 | 4.2 | 350 – 400 | 0.1 |
| 30 – 35 | 4.4 | 400 – 450 | 0 |
| 35 – 40 | 3.5 | 450 – 500 | 0.1 |
| 40 – 45 | 2.9 | 500 – 1000 | 0.2 |
| 45 – 50 | 4.9 | 1000 – 1500 | 11.3 |
| 50 – 75 | 15 | ||
| 75 – 100 | 13.3 |
Trapped:
Particles that collide with the face-tent mask, are drawn out into the suction flow, or collide with the patient’s face and become trapped there. These particles are considered to pose no additional risk to care-givers, as these surfaces would be considered contaminated and only touched with protective measures.
Escaped:
Particles that remain airborne or exit the computational domain of the simulation.
Subsequent Modeling
Subsequent modeling to evaluate the possible synergy of addition of a surgical mask along with the use of the FELIX-1 was conducted. In that model, the same patient representative geometry, FELIX-1 and HVNI mechanics were employed. The addition of a model of a simple Type-1 surgical facemask (the capture characteristics of the mask alone are previously described), was implemented for the purpose of estimating the potential particle capture within the region of the FELIX-1 or evacuated by the suction (Figure 4a). [19] A further simulation was performed with a mask placement simulated over the opening of the FELIX-1 patient interface (Figure 4b). Proportion of particle capture was reported for the purpose of estimating the degree of additive protection, if any.

RESULTS
Characterization of Particle Dispersion
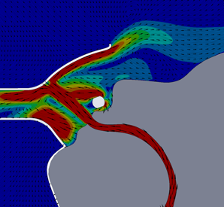
For this CFD simulation of a single human anatomic rendering being subjected to HVNI at 40 L∙min-1 with a FELIX-1 in place, up to 97% of particle mass in the simulation remained trapped within the FELIX-1 facemask interior space or evacuated to the suction system. The single anatomy model suggested that for patients exhaling (the portion of the ventilatory cycle with greatest particle escape), the flow dynamics showed the FELIX-1 with suction applied reduces the flow velocity and redirects a portion of the flow into the suction port while a portion of the flow escapes the mask (Figure 5). The direction change causes a portion of the particles to impact the mask where they remain trapped. The remaining particles are either drawn into the suction system or escape the mask with a reduced velocity.
Temperature Modeling at the FELIX-1 Interface
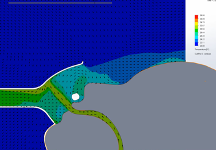
Temperature of the region inside the FELIX-1 mask was studied. During the breathing cycle the temperature of the mask interior varies as expected. During inspiration (Figure 6), the relatively cool room air (ambient air simulated at 20℃) is drawn into the mask by the suction system, thus reducing the temperature of the gases in contact with the patient for a portion of the breathing cycle. By contrast, a surgical mask used in conjunction with HVNI therapy will have a continuous temperature close to 37℃.
Effect of Suction Airflow on Simulated Particle Dispersal
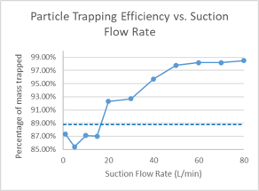
Clinical suction systems typically target a minimum gas flowrate induced by the vacuum of approximately 20 L∙min-1. The effect of suction flowrate was modeled, and the amount of particle capture was related to suction induced flowrates, with suction flowrates of at least 20 L∙min-1 associated with significant capture of particle mass. Capture continues to improve at higher suction flowrates, tending to plateau in this model above 97% of virtual particle mass accounted for in the mask at suction flowrates above 50 L∙min-1 from the vacuum system (Figure 7).
Subsequent Modeling
Subsequent modeling was performed simulating concomitant use of both the FELIX-1 and a simple surgical facemask, both affixed on the model patient’s face with HVNI therapy at 40 L∙min-1 being simulated.
The prior simulation showed the FELIX-1 nominal capture in the simulation to be approximately 97% of particle mass. The addition of the surgical mask inside the FELIX-1 in this single anatomy model actually had a lower capture proportion (96%). The FELIX-1 with the mask over the opening of the facial interface did not show any improvement in capture (97%). The simulation also showed greater heat being held at the simulated face of the patient during exhalation, for both the mask under the FELIX-1 interface as well as for the mask over the aperture of the interface (Figure 8).

DISCUSSION
This in silico modeling adds potentially meaningful information for clinicians who may want to use the FELIX-1 scavenger system in a clinical context. The simulation suggests that particle mass dispersal could be reduced as compared to no mitigating technology in place, with a large proportion of that mass captured in the vicinity of the patient’s face. The details of the simulation also show that the particles which escape the mask are almost exclusively small (<5 μm) airborne aerosol particles. The actual transmissivity of such small particles remains to be demonstrated.6 The amount of particle mass captured in this simulation of the FELIX-1 mask was not augmented when used in conjunction with a simulated surgical mask placed over either the model patient’s face or over the model FELIX-1 opening. This may be due to the fact that the addition of a surgical mask was modeled to include leaks. These leak points were described previously and were designed to permit analysis of a plausible clinical scenario in which the mask is poorly fit (i.e., leaks at the bridge of the nose, and sides of the mask). These leaks provide a small area for gas flow to escape the field of the mask, imparting greater velocity to the exiting gas flow and any gas-borne particles entrained it that flow. This greater velocity for these particles directed out of the field of the FELIX-1 permitted these high-momentum particles to escape the model. Also, the intentional ‘leaks’ built into the system may in an actual physical sense be overcome by extraordinary means at sealing the mask to a patient’s face. Such efforts, however, seem unlikely in a normal clinical context.
The capture within the FELIX-1 is likely due to two things – the barrier geometry of the FELIX-1 interface positioned in direct line of exhaled gas flow exiting the patient, and the application of the negative pressure field due to the instillation of suction for the FELIX-1. This ability to capture and clear particle mass was affected by the amount of suction-related airflow applied to the opening of the FELIX-1 connector to the suction system (i.e., away from the patient). Hospital suction systems are typically required to provide a minimum of 20 L∙min-1 of gas flow under suction. In a recent test at a major teaching hospital in New York, New York, the measured gas flow related to the suction system attached to a physical FELIX-1 system was approximately 30 L∙min-1 (unpublished report). These simulations suggest that generally, the greater the suction flow, the higher proportion of particle capture possible. However, the higher suction flow to extreme levels could prove challenging when applied across a large number of FELIX-1 systems attached to a single vacuum system in the hospital.
Although this simulation suggests theoretically that the FELIX-1 interface may be capable of capturing larger (>5μm) particles, the actual use in clinical medicine must be carefully considered. This in silico analysis has several important limitations. First, the simulation is performed on geometry derived from a single subject anatomy. Different anatomic characteristics may provide variation in the actual dynamics of flow into and exiting the FELIX-1 scavenger. Second, the simulation studied only a secure fitting FELIX-1 model. Security of the facemask may be challenging, especially with a patient engaged in moving in bed (e.g., when self-proning). Third, the simulation assumes a constant vacuum airflow. Such airflow may be subject to variability in an actual clinical environment, as devices come on and off-line to the hospital vacuum system. Fourth, the model only accounted for tidal breathing, and did not study the likely effect of high velocity transient behaviors likely from patients (e.g., coughing and sneezing). Fifth, the model did not consider room airflow dynamics, although such low-velocity airflow effects are likely negligible. Finally, the in silico model is just that – a theoretical extrapolation of gas/particle behaviour (albeit based on well established physics).
With such cautions in place, it is imperative clinicians managing infectious patients continue to follow established guidelines regarding use of protective apparatus and environments. Scrupulous attention to personal safety, including use of personal protective equipment, as well as employing negative pressure environments when available is essential. If an actual negative pressure space such as FELIX-1 is employed in patient care, the clinician also needs to recognize that the space within the FELIX-1 interface may have captured 97% of the particle mass leaving the patient. As such, that space becomes a likely ‘hot’ zone of particle deposition and potential transmission to staff/others.
CONCLUSION
Clinical interventions to mitigate particle dispersion during High Flow therapies delivered to potentially infectious patients remain important. Mitigation begins with proper selection of therapies for the patient, along with strict adherence to the use of personal protective equipment and environmental controls when possible (e.g., negative pressure rooms). As with a simple surgical mask, the FELIX-1 may offer clinicians another tool to help mitigate particle dispersal in the patient room. These initial computer simulations may be helpful in guiding future in vitro modeling and must be interpreted cautiously in the context of clinical management of potentially infectious patients. Care must be given to any physical deployment, with rigorous attention paid to alternative standard means of controlling viral spread, to the integrity of high suction-related flowrates in the mask, and proper placement of a mask on the patient’s face. These simulations suggest FELIX-1 is likely capable of generating a region of relative negative pressure in the region of the patient’s face.
FINANCIAL DISCLOSURE/ CONFLICT OF INTEREST
Both DUNGAN and LEONARD are paid employees and shareholders of Vapotherm, Inc. Further both have patents issued which have been assigned to Vapotherm, Inc.
ACKNOWLEDGMENT
The authors acknowledge the invaluable advice and counsel of Felix Khusid RRT-ACCS, NPS, RPFT, FAARC, FCCM, FCCP, ATSF, who contributed the original idea for the device, as well as technical advice during the conduct of the simulation. The authors also acknowledge and thank the peer-reviewers (Michael McQueen, MD and Ronald DeBellis, PharmD, FCCP), during preparation of this white paper.
REFERENCES
[1] World Health Organization. Clinical management of severe acute respiratory infection when Novel coronavirus (2019-nCoV) infection is suspected: Interim Guidance. WHO reference number: WHO/nCoV/Clinical/2020.3 13 March 2020.
[2] Alhazzani W, Moller MH, Arabi YM, et al. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Critical care medicine. 2020;PREPUBLICATION.
[3] Respiratory care committee of Chinese Thoracic S. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;17(0):E020.
[4] Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. [Recommendations for critically ill patients with COVID-19]. Medizinische Klinik, Intensivmedizin und Notfallmedizin. 2020.
[5] WHO. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020.
[6] World Health Organization. Tranmission of SARS-CoV-2: Implications for Infection Prevention Procedures. WHO Scientific Brief. 2020;9 July 2020.
[7] WHO. Infection prevention and control of epidemic and pandemic prone acute respiratory infections in health care. Geneva, Switzerland 2014.
[8] Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797.
[9] England DoHaSC-PHW-PHANI-HPS-PH. Guidance for infection prevention and control in healthcare settings: Adapted from Pandemic Influenza Guidance for Infectoin Prevention and Control in Healthcare Settings 2020. 2020.
[10] Gralton J, Tovey E, McLaws ML, Rawlinson WD. The role of particle size in aerosolised pathogen transmission: a review. J Infect. 2011;62(1):1-13.
[11] Tang JW, Li Y, Eames I, Chan PK, Ridgway GL. Factors involved in the aerosol transmission of infection and control of ventilation in healthcare premises. J Hosp Infect. 2006;64(2):100-114.
[12] Yang S, Lee GW, Chen CM, Wu CC, Yu KP. The size and concentration of droplets generated by coughing in human subjects. J Aerosol Med. 2007;20(4):484-494.
[13] Xie X, Li Y, Sun H, Liu L. Exhaled droplets due to talking and coughing. J R Soc Interface. 2009;6 Suppl 6:S703-714.
[14] Kwon SB, Park J, Jang J, et al. Study on the initial velocity distribution of exhaled air from coughing and speaking. Chemosphere. 2012;87(11):1260-1264.
[15] Cheung JC, Ho LT, Cheng JV, Cham EYK, Lam KN. Staff safety during emergency airway management for COVID-19 in Hong Kong. Lancet Respir Med. 2020.
[16] Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during noninvasive ventilation via helmets and a total facemask. Chest. 2015;147(5):1336-1343.
[17] Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks. The European respiratory journal. 2019;53(4).
[18] Leonard S, Atwood CW, Jr., Walsh BK, et al. Preliminary Findings of Control of Dispersion of Aerosols and Droplets during High Velocity Nasal Insufflation Therapy Using a Simple Surgical Mask: Implications for High Flow Nasal Cannula. Chest. 2020.
[19] Leonard S, Strasser W, Whittle JS, et al. Reducing aerosol dispersion by high flow therapy in COVID-19: High resolution computational fluid dynamics simulations of particle behavior during high velocity nasal insufflation with a simple surgical mask. Journal of the American College of Emergency Physicians Open. 2020;n/a(n/a).