New Analysis Suggests Vapotherm High Velocity Therapy May Be as Effective as Noninvasive Positive Pressure Ventilation (NiPPV) Among Studied Hypercapnic Patients
In April 2020, Doshi and colleagues published the results of a subgroup analysis in Heart & Lung titled “The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: a subgroup analysis.” The analyses examined the hypercapnic sub-population of a multi-center, randomized, controlled, non-inferiority trial. The larger multi-center trial found that high velocity therapy—in the study referred to as High Velocity Nasal Insufflation (HVNI)—had similar outcomes to Noninvasive Positive Pressure Ventilation (NiPPV) in adults presenting with undifferentiated respiratory distress in the Emergency Department (ED). [1] Similarly, the subgroup analysis concluded that high velocity therapy may provide ventilatory support comparable to NiPPV in this hypercapnic sub-population. [2]
Initial Randomized Controlled Trial and Subgroup Selection
The larger multi-center trial on which the subgroup analysis draws, randomized ED patients requiring non-invasive respiratory support to be treated with either NiPPV or high velocity therapy. The primary outcome was therapy failure at 72 hours following enrollment. Therapy failure was indicated by intubation or all-cause arm failure, as clinician-directed crossover to alternative therapy was an option. The subgroup selection for this analysis was established during the original study design and included those patients who either had: 1) a primary diagnosis of acute exacerbation of COPD (AECOPD) or 2) acute hypercapnic respiratory failure at discharge. 34 patients randomized to high velocity therapy and 31 to NiPPV met this a priori subgroup criteria.
Baseline Characteristics
The patient baseline characteristics between the two groups differed in only two categories: therapy setup time and initial respiratory rate. The therapy setup time for NiPPV was 6 minutes vs 10 minutes for high velocity therapy. The initial respiratory rate for NiPPV was 28 breaths/min and 32 for high velocity therapy. All other characteristics were similar between the study arms.
Primary Outcomes
Primary outcomes examined were the PCO2 and pH changes over time. They were found to be comparable, indicating that high velocity therapy, among patients in this study, provided ventilatory support similar to NiPPV.
Secondary Outcomes
Regarding the patients’ physiological measures over time, they trended similarly between the two therapies, as seen in Table 1:
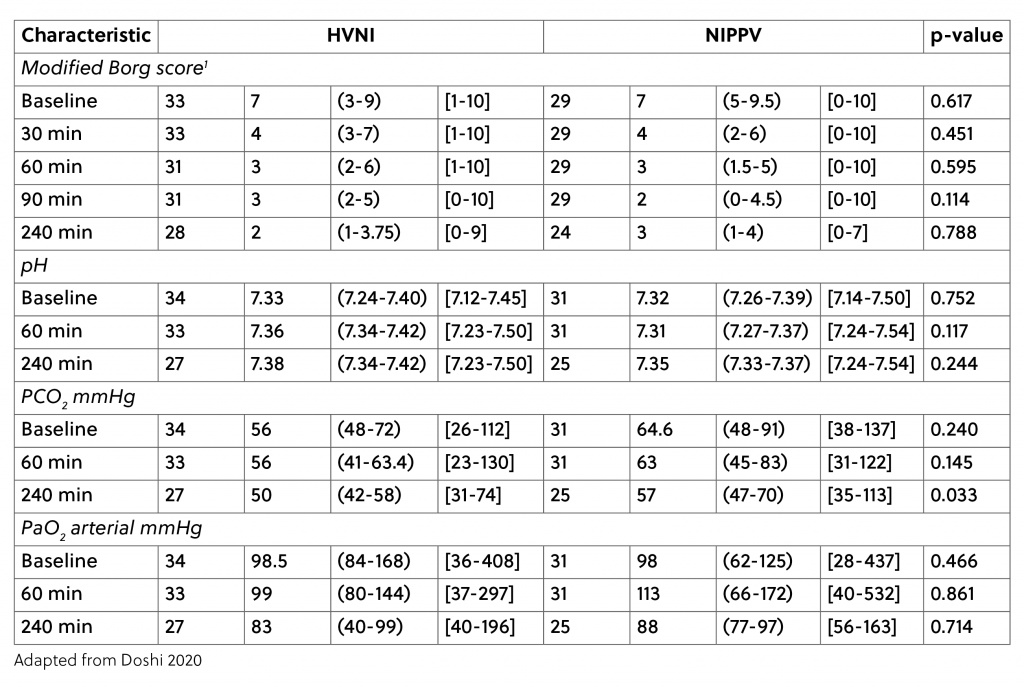
Furthermore, there was also no significant difference in intubation rate (16.1% NiPPV, 5.9% high velocity therapy) and in all-cause therapy failure (25.8% NiPPV, 23.5% high velocity therapy). The salvage rate for both groups following crossover was 75%. For both therapies, if they failed, half the time it was due to failure to ventilate. For the NiPPV arm, the remaining half of failures occurred due to an inability of the patients to tolerate the modality. It is interesting to note that none of the high velocity therapy failures were due to an inability to tolerate the therapy.
Given that high velocity therapy salvaged 75% of the patients intolerant of NiPPV, the data suggest that a larger follow-on trial may be valuable to help determine if high velocity therapy could be an NiPPV alternative to try in this patient population before using intubation.
Length of Stay
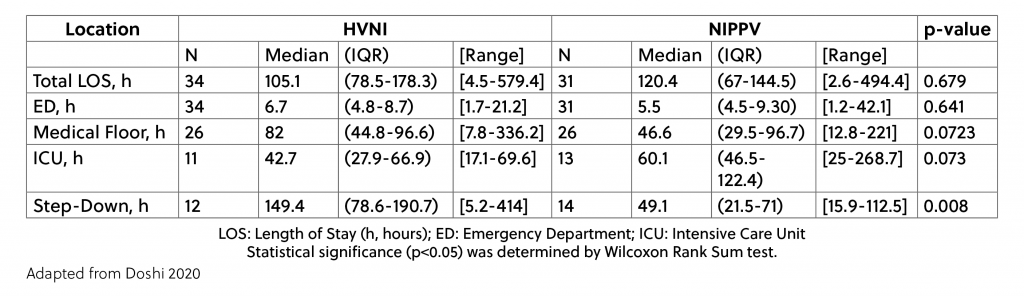
The length of stay (LOS) overall was also similar between the study arms, however, the breakdown of LOS per hospital area showed some differences. These high velocity therapy patients spent less time in the ICU than the NiPPV patients, but more time on the Medical Floor and in Step-Down units. Table 2 shows the breakdown.
Limitations
Limitations of the analysis include the fact that the original trial was not powered for this subgroup analysis. Additionally, although not statistically significant, the baseline PCO2 was lower in the high velocity therapy arm which may have some clinical relevance despite it not being a statistically significant difference. Despite these limitations, the authors conclude that the results of these analyses suggest that high velocity therapy “may be considered another noninvasive ventilation therapy, that is available in managing patients with acute hypercapneic respiratory failure” and call for further research.
The study was conducted on Vapotherm high velocity therapy, which at the time of publication is the only device capable of delivering high velocity therapy across all interface sizes.
Download an eBook on treating COPD exacerbations with High Velocity Therapy
REFERENCES
[1] Doshi, Pratik et al. High-Velocity Nasal Insufflation in the Treatment of Respiratory Failure: A Randomized Clinical Trial. Annals of Emergency Medicine, 2018. https://www.ncbi.nlm.nih.gov/pubmed/29310868
[2] Doshi PB, Whittle JS, Dungan G, et al. The ventilatory effect of high velocity nasal insufflation compared to non-invasive positive-pressure ventilation in the treatment of hypercapneic respiratory failure: A subgroup analysis. Heart & Lung. 2020 Epub Ahead of Print.