Vapotherm High Velocity Therapy
COVID-19 Resource Center
Vapotherm high velocity therapy provides mask-free non-invasive ventilatory support. It’s a trusted front-line tool for respiratory distress – including hypoxemia, hypercapnia, and dyspnea.
As a refined form of high flow nasal cannula, it can be used to provide high flow oxygenation. As an advanced respiratory tool, it can also help hypercapnic and hypoxemic patients avoid endotracheal intubation.
This page was compiled by the doctors and clinical researchers in our Medical Education and Science and Innovation teams to address frequently asked questions regarding Vapotherm high velocity therapy with COVID-19 patients. We are committed to updating this page as new information becomes available.
Our COVID-19 Clinical Hotline
We’re here for you.
In addition to our team of clinical managers supporting you locally, we’ve opened a 24/7 hotline for questions regarding the use of Vapotherm high velocity therapy and COVID-19.
- For questions and support specific to COVID-19, contact the COVID-19 hotline at 1-833-333-2770.
- For Customer Service, such as order status and disposables, call 1-855-320-4506.
- For 24/7 Technical Support, including troubleshooting, call 1-866-566-2652.
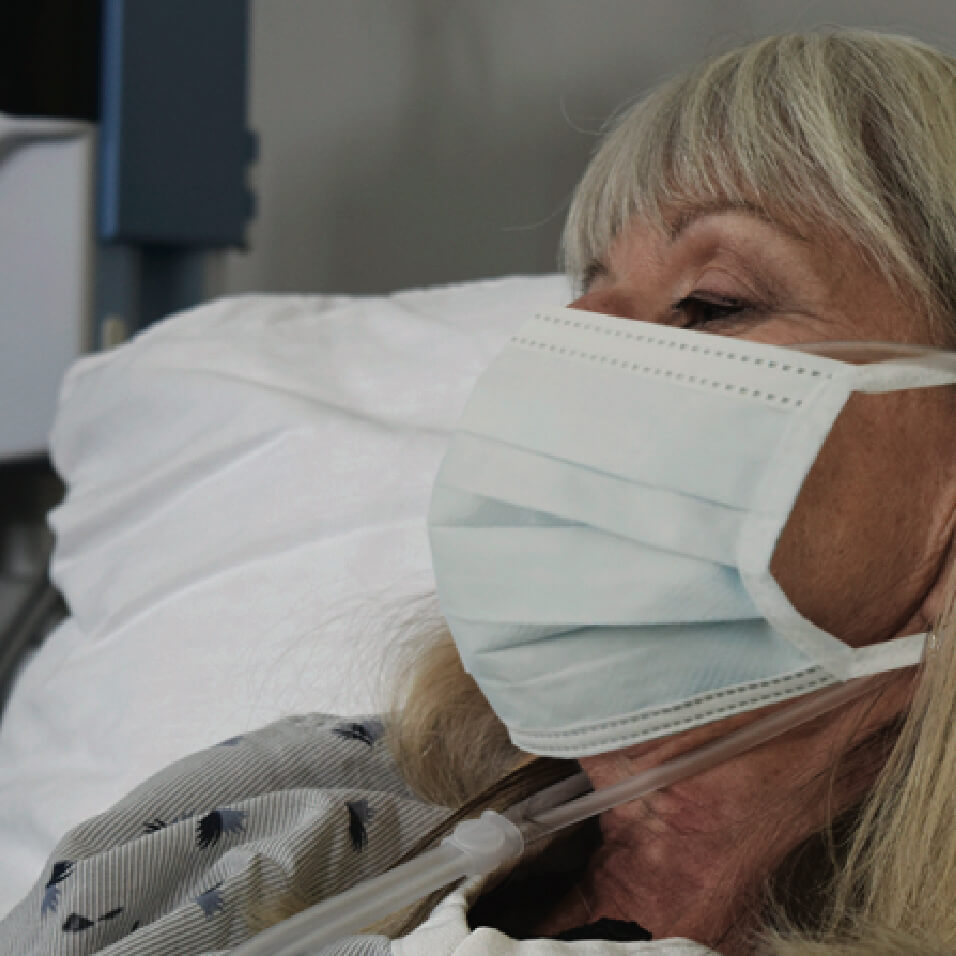
Precautions when Treating with High Velocity Therapy
The World Health Organization (WHO) and the Centers for Disease Control (CDC) have recommended precautions be taken when treating patients on aerosol generating procedures. Their recommendations include that healthcare workers wear particulate respirator (N-95, FFP or equivalent), protective glasses, gown, and gloves, and that they perform procedures in an adequately ventilated room, preferably an Airborne Infection Isolation Room (AIIR) or Negative Pressure room.
After fitting the cannula to the patient, apply a surgical mask over the nose and mouth of the patient then initiate treatment.

Area Vice President Clinical
On-demand Webinar: COVID-19 Patients on High Velocity Nasal Insufflation – Considerations & Transmission Protection
John Walsh presents the highly relevant course about “COVID-19 Patients on High Velocity Nasal Insufflation – Considerations and Transmission Prevention.” In this course, you will:
- Review COVID-19 symptoms
- Review COVID-19 transmission risks
- Discuss HVNI in the context of COVID-19 management
- Overview the proper protective measures to reduce transmission risk during HVNI
This course is AARC accredited for 1 CEU under the Clinical Practice (CLP) category
Understanding the Risk of Transmission to Healthcare Workers
All respiratory care represents a risk of aerosol generating procedures. High velocity/high flow therapy, when properly fitted and applied, is associated with a low risk of airborne transmissions. [1] [2] [3]
The application of a surgical mask placed over the nose and mouth of the patient receiving high velocity therapy substantially reduces the spread of airborne particles by reducing their velocity and trapping the particles.
With the mask applied, the dispersion captured is similar to that of patient breathing.
A study examining the risk of pathogen dispersal during high flow therapy found “that dispersal was limited to the proximal area of the face and nasal cannula, suggesting that high-flow therapy does not increase the risk of droplet and contact infection.” [4]
A meta-analysis assessed the risk of transmitting SARS to healthcare workers, due to various interventions. Intubation and associated procedures carry the most risk. High flow therapy actually trended towards reduced risk of transmission. This suggests that using high flow therapy to avoid intubation might reduce transmission risk. [5]
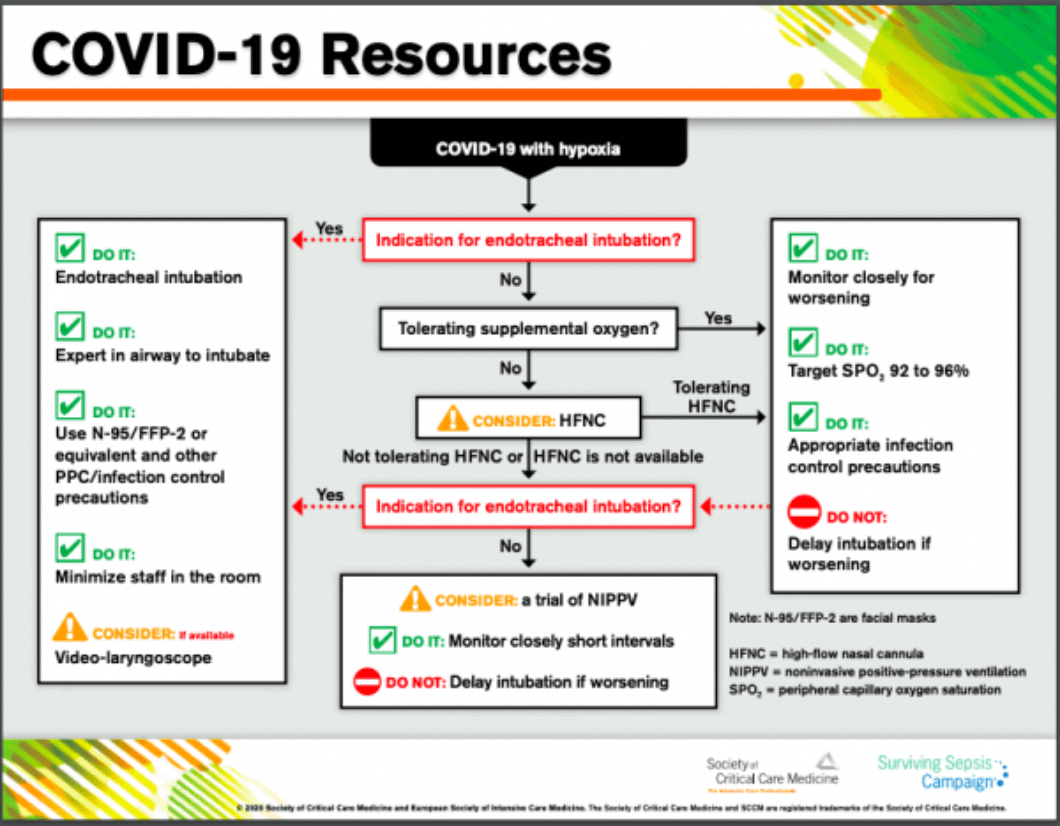
Guidelines Update from SCCM
The Society for Critical Care Medicine (SCCM) includes high flow nasal cannula in its recently released COVID-19 guidelines to manage critically ill adults with COVID-19 in the intensive care unit (ICU). The Centers for Disease Control and Prevention (CDC) has also shared these guidelines on their webpage.
Click here to access the infographic an disasterd complete guidelines from SCCM.
To see more guidelines and suggestions from around the world, read our recent article spanning China and Italy to US Department of Defense.

Availability of Vapotherm Systems & Patient Circuits
Vapotherm is committed to supporting the needs of all our hospitals, healthcare professionals, and patients.
Nearly all of our supply chain is located within the United States, including our manufacturing facility. We’ve increased our production capabilities, and taken precautions for the health and safety of our manufacturing team, such as training, environmental spacing, and daily temperature checks, in order to continue building Precision Flow units and patient circuits.
Ensure you have enough units and adjusted PAR levels for patient circuits, and work with our customer service team at 1-866-566-2652 if you have any questions regarding available inventory and ordering.
Velocity – Ep 8 – Respiratory Directors Share COVID-19 Experiences
[FELIX-1 White Paper] – Computational Fluid Dynamic Modelling of Particle Capture: Possible Use of a Modified Face-Tent as a Facial Scavenger

FELIX-1: Creating a Localized Area of Negative Pressure for COVID-19 Patients

We’re here for you. A COVID-19 update from CEO Joe Army
Frequently Asked Questions
Is Vapotherm high velocity therapy an aerosol generating procedure?
Use of any equipment which supports ventilation or oxygenation has the potential to generate aerosol, and the possibility of transmitting pathogens. High velocity therapy and other non-invasive ventilation modalities present such challenges, and require appropriate use of PPE in the care of the patients.
Can I use a surgical mask over the patient’s mouth while the patient is on therapy?
Yes. Vapotherm has conducted preliminary work with computational fluid dynamic modeling of an adult patient on high velocity therapy with a surgical facemask covering the mouth and nose with the cannula in place. The results suggest that the surgical mask receives the overwhelming majority of the flow in this model, and acts to diffuse the flow. This practice is also consistent with guidance from the Chinese Thoracic Society. [6]
The greatly lowered velocity of this gas stream allows for the mask to capture the particles as intended. The resulting outflow patterns from patients may resemble those from patients simply spontaneously breathing while wearing such masks without high velocity therapy.
In these simulations, the most obvious source of particulate movement was through leaks in the mask. Therefore, we suggest paying close attention to securing the mask on the patient to minimize mask leakage.
Further work continues, and we will shortly be providing guidance on the actual geometry of deposition around the patient.
How do we disinfect/clean the unit between use?
Refer to your device’s instructions for use for full cleaning instructions in addition to policies and procedures in place by your hospital. Our instructions for use call for Super Sani-Cloth, which has been listed by the EPA as effective against coronavirus. Ensure the single-use disposable patient circuit is disposed of appropriately after use. Do not attempt to reuse the single-use patient circuit.
Refer to the instructions for use for cleaning instructions.
Is high velocity therapy an effective treatment for acute hypoxemic respiratory distress?
Yes. Clinical evidence supports use of high flow/high velocity therapy as an effective tool to treat hypoxemic respiratory failure. It has lower intubation rates to conventional oxygen and similar intubation rates to NiPPV. [7]
What precautions can I take while using high velocity therapy?
Use a type-1 surgical mask covering the patient’s nose and mouth to reduce dispersal.
Although the infection risk from high velocity therapy is low, the World Health Organization (WHO) and the Centers for Disease Control (CDC) recommend precautions be taken when treating patients on aerosol generating treatments, including PPE. WHO also suggests close monitoring of patients to evaluate any need for escalation.
Their recommendations include that healthcare workers wear particulate respirator (N-95, FFP or equivalent), protective glasses, gown and gloves, as well as the use of an Airborne Infection Isolation Room (AIIR) or Negative Pressure room.
SOURCES: [1] Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: Interim Guide, Page 6 January 28 2020. [2] Leung CCH, Joynt GM, Gomersall CD, et al. Comparison of high-flow nasal canula versus oxygen face mask for environmental bacterial pneumonia patients, a randomized controlled crossover trial. J Hosp Infect 2019: 101:84-87. [3] Hui DS, Chow BK, Lo T, et al. Exhaled air dispersion during high-flow nasal cannula therapy versus CPAP via different masks, Eur Respir J 2019,53. [4] Kotoda M et al., Assessment of the potential for pathogen dispersal during high-flow nasal therapy, Journal of Hospital Infection, https://doi.org/10.1016/j.jhin.2019.11.010. [5] Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J (2012) Aerosol Generating Procedures and Risk of Transmission of Acute Respiratory Infections to Healthcare Workers: A Systematic Review. PLoS ONE 7(4): e35797. doi:10.1371/journal.pone.0035797 [6] Respiratory care committee of Chinese Thoracic S. [Expert consensus on preventing nosocomial transmission during respiratory care for critically ill patients infected by 2019 novel coronavirus pneumonia]. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2020;17(0):E020. [7] Ou X, Hua Y, Liu J, Gong C, Zhao W. Effect of high-flow nasal cannula oxygen therapy in adults with acute hypoxemic respiratory failure: a meta-analysis of randomized controlled trials. CMAJ 2017;189:E260-E7.